CO2MUCH - Carbon Civilisation: Sources of CO2

Overview
Overview
Keywords: CO2, combustion, balanced equation
Disciplines: Physics, chemistry, science and technology
Age level of the students: 13-15
Time frame: 3-5 lessons
![]()
![]()
![]()
Introduction to the topic
First, the teacher explains the chemical composition of different fossil fuels such as coal, wood and petrol using molecular models or virtual models of methane, octane or another hydrocarbon molecule. Students are asked to find out the chemical formula. They can use a digital activity.
The teacher also asks the students to think about the products that can be formed during combustion. The students can discuss in groups to predict what the products of the combustion of coal, wood and gasoline will be. They explain their ideas to the class.
Students use the carbon civilisation H5P presentation to answer questions about carbon combustions and to verify their hypothesis. This presentation provides a brief historical overview of the use of fossil fuels throughout human history and the role of carbon and combustions on the greenhouse effect.
Students make a link between human activities, fossil fuels combustion, carbon dioxide and climate change.
Identification tests
To recognise a material, chemists use identification tests.
Depending on the level of the students, the teacher either asks the student groups to propose an experimental protocol to verify the production of carbon dioxide during combustion of fossil fuels, or simply gives them the protocol. In the first case, each group can present the protocol to be validated by the classroom and the teacher. They can use the test identification list.
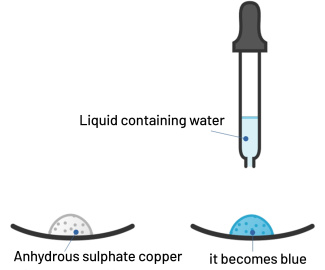
Water identification test
To identify the presence of water (H2O), the anhydrous copper sulphate test is used. Anhydrous copper sulphate is whiteish. It turns blue when in contact with water.
Protocol: pour a few drops of liquid to be tested on anhydrous copper sulphate.
Result: if the anhydrous copper sulphate turns blue, the liquid contains water.
Oxygen identification test
To identify oxygen (O2), we use the glowing splint test. Oxygen revives a glowing splint.
Protocol: approach a glowing splint (an ember).
Result: if the splint ignites, the gas is oxygen.

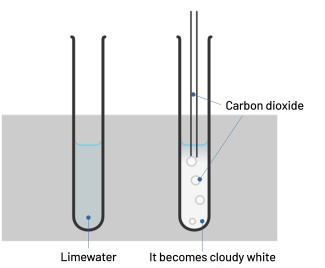
Carbon dioxide identification test
To identify carbon dioxide (CO2), we use the limewater (or calcium hydroxide) test. Carbon dioxide reacts with limewater: a whitish precipitate appears and the limewater becomes cloudy.
Protocol: put the limewater in contact with the gas to be tested.
Result: if the limewater becomes cloudy, the gas is carbon dioxide
Combustion of carbon
Students experiment to demonstrate the production of carbon dioxide during the combustion of fossil fuels. They study the combustion of carbon. The laboratory activity consists of burning a charcoal stick and identifying the gas formed during the combustion as carbon dioxide using limewater.
We want to implement the combustion of carbon in pure oxygen to verify that it produces carbon dioxide, the main greenhouse effect gas.
Materials
- Piece of charcoal held by an alligator clip attached to a cork
- Flask full of pure oxygen
- Lighter
- Syringe
- Test tube
- Flask of limewater
Protocol 1: combustion of carbon (scheme: step 1 and 2)
- Attach the charcoal tip to the alligator clip and cork
- Make the charcoal tip glow
- Unscrew the oxygen flask and insert the charcoal into the flask (see diagram).
- Do not lift the cork
- Write down your observations
Protocol 2: gas identification (scheme: step 3 and 4)
- Pour about 2 cm3 of limewater into the test tube.
- Use the syringe to extract the gas formed in the flask (scheme: step 3).
- Inject the gas into the limewater in the test tube (scheme: step 4).
Questions
- What are the two reagents of the combustion?
- What is the product of the combustion?
- Write down the chemical balance of the combustion

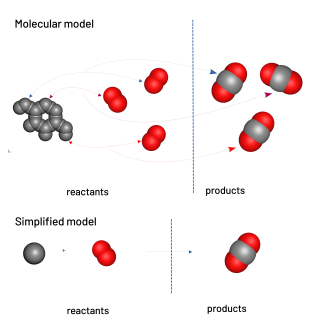
Modelling the combustion of carbon
The teacher models the transformation from a microscopic point of view using the molecular models and the balance equation. Students can then write the balance of the chemical reaction.
chemical balance: carbon + oxygen → carbon dioxide
balanced chemical equation: C + O2 → CO2
Exercise: combustion of carbon
Students can verify their understanding of the combustion of carbon and methane by using an interactive video on combustion.
Combustion of methane
Depending on the material, students or teachers can experiment the combustion of methane. However, they can analyse a video of an experiment and answer some questions using an interactive video.
We want to implement the combustion of methane in the air to verify that it produces carbon dioxide, the main greenhouse effect gas.
Materials
- A Bunsen burner providing methane
- 2 Erlenmeyer flasks
- Anhydrous copper sulphate
- A flask of limewater
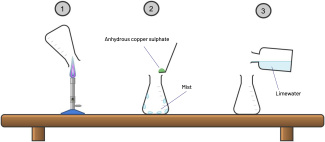
Protocol 1a: combustion of methane (scheme: step 1)
- Put the Erlenmeyer flask above the flame of the Bunsen burner.
Protocol 1b: mist identification (scheme: step 2)
- Put some anhydrous copper sulphate in the Erlenmeyer flask on the mist.
Protocol 2a: combustion of methane (scheme: redo step 1)
- Put the second Erlenmeyer flask above the flame of the Bunsen burner.
Protocol 2b: mist identification (scheme: step 3)
- Pour some limewater in the Erlenmeyer flask.
- Shake the flasks.
Questions
For each protocol, describe what you observe and draw the appropriate conclusion.
Modelling the combustion of methane
The teacher models another transformation.
chemical balance: methane + oxygen → carbon dioxide + water
balanced chemical equation: CH4 + 2 O2 → CO2 + 2 H2O
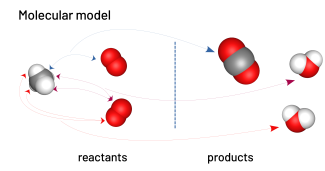
Students can verify their understanding of the combustion of methane by using an interactive video of the combustion.
Conclusion
The students can confirm that combustion of fossil fuels due to human activities produces carbon dioxide because these fuels contain carbon atoms. They can also create a mind map of the knowledge acquired during these different activities.
Optional experimentation can be conducted with wood or petrol to demonstrate the production of carbon dioxide. The protocol is the same as for methane combustion.
Students practise writing balanced chemical equations using Phet Interactive Simulations
Authors of CO2MUCH - Think Global, Act Local: Elena Poncela Blanco (ES), Philippe Mancini (FR)
Links to forward to your students
Simulation by PhET Interactive Simulations, University of Colorado Boulder, licensed under CC-BY-4.0; https://phet.colorado.edu
Share this page


