Finding Water in the Universe

Overview
Overview
Keywords: astrobiology, meteorites, solar system, water
Subjects: chemistry, physics, biology, astronomy
Other disciplines: mathematics, theatre
Age level: 8–12
Time frame: 5 lessons (30 minutes framework and 45–60 minutes experimental work each)

This project by Juan Antonio Prieto Sánchez and María Pilar Orozco Sáenz was presented at Diverciencia, the science fair of Algeciras, Spain. It was also shown at the Science on Stage festival 2022 in Prague as part of an “Astrobiology in Primary School“ project which was awarded the first prize in the category “Science for the Youngest“. The photos on this page were taken during the implementation of the project with students of Year 5 and 6 from Colegio Huerta de la Cruz, Algeciras.
Conceptual Introduction
Water is one of the most abundant molecules in the universe. Due to the pressure and temperature conditions of the interstellar medium, water is usually found in a solid or gaseous state outside the Earth.
Earth is an exceptional planet. It is the only planet we know in which life exists. This is due to the large amount of liquid water that it contains. The origin of water on Earth is still unknown, and it is considered a very controversial issue.
Life on our planet depends on and is intrinsically linked to water due to its physical and chemical properties. To study the origin of water on our planet is to learn about our own existence. When scientists search for living organisms outside our planet, they investigate the presence of water.
Required materials, hardware and software
- Materials (chemicals, lab glassware materials, household items)
- Mobile phones
- An application to measure the intensity of light
- Computers
- Internet connection




Part 1: The origin of terrestrial water
Recent studies suggest that carbonaceous chondrites raining on Earth could have been the origin of water and organic matter in our newborn planet.
Carbonaceous chondrites are primitive meteorites that form in oxygen-rich regions of the solar system. They represent 3% of all meteorites collected after falling to Earth. They are important because of the insights they provide into the early history of the solar system.
This first water must have been trapped in the primitive atmosphere due to the temperatures of the planet. Additionally, volcanic activity released large amounts of water vapour.
As the Earth cooled down the oceans formed. However, these oceans consisted of fresh water. Little by little, due to the cycle of water, the oceans became salty.
In this first part, we will experiment how water might have reached Earth. We will also see that sometimes solid matter can contain water, and we will explain, with the use of drama, how oceans became salty.
Materials
Plastic film
Soil
Two trays
Method
- With the help of plastic film, we simulate the meteorites by making small balls with clay, some soil and water. Then we place them in the freezer overnight to solidify.
- We prepare two trays with soil. We throw “the frozen meteorites” in one of the trays. The tray which has had the impacts, increases in mass, when compared to the other tray.
- Once “the meteor shower” is over, we cover that tray with plastic film and place it in the sun. The water evaporates and gets condensed on the film, thus simulating the existence of water in the atmosphere.
Materials
Paper
A large glass or metal container with a lid
Matches/lighter
Method
- We burn some paper in a container.
- We place ice on the lid and hold it over the fire so that the heat will reach it, but not the flames.
- After a few seconds, we are able to observe the water droplets that form on the lid.
Important note: Students need to work under the supervision of the teacher and in accordance with fire safety regulations.
Materials
Ballons of different colours
Two large boxes
Method
This is an activity which can be organised outside, e. g. in the school yard. We put a box (oceans, box A) with blue balloons inside (water molecules) on one end of the yard and another box (mountains, box B) with coloured balloons (salted molecules) on the other end. The students (clouds and rivers) collect blue balloons from box A and take them (evaporation, cloud formation) to box B (mountain). Once there, they pick coloured balloons without releasing the blue ones (precipitation). They then go back to box A (surface runoff), release the coloured and blue balloons (mouth of a river), and go back to box B with only blue balloons, completing the cycle. After several cycles, it can be observed that now, there are many coloured balloons in the ocean box that at the beginning only contained blue balloons: the ocean has become salty.
Video links
Impressions of the activity being carried out at a Spanish primary school can be found here:

Activities 1 and 2

Part 2: Lunar water
NASA has confirmed the presence of water on the Moon. Water ice is found in “cold traps”, large, shadowed regions in the poles of our satellite, where temperatures are extremely low (‑163 oC). This is the case of the Clavius crater.
The presence of water is fundamental for future human spaceflight. First of all because water is essential for living organisms and humans cannot survive more than 4–5 days without drinking water, but also because the hydrogen contained in the water molecule could be used as fuel on the Moon.
In this part, we will investigate how water can be isolated from different mixtures.
Materials
Crucibles
Laboratory scale
Oven
Wet sand
Method
We weigh a sample of wet sand. We dry it in the oven for an hour and we weigh it again. The difference in weight accounts for the water.
|
|
Weight [g] |
|
Crucible |
|
|
Hydrated sample |
|
|
Dried sample |
|
|
Water |
|
|
% of water in the sample |
|
Materials
Laboratory scale
A sample of salted water
Flask
Tripod
Gauze mat
Bunsen burner
Method
We weigh a sample of salted (sea) water. We place it over the burner and boil it until all the water has evaporated. The evaporated water can be condensed in another flask if wanted. Finally, we weigh the flask to calculate the amount of salt.
| Weight [g] | |
| Flask + salted water | |
| Flask after evaporation | |
| Salt | |
| Water | |
| % of salt in the sample |
Materials
A sample of salted water
Sand
Beaker
Funnel
Filter paper
Tray
Method
We weigh a sample of salted (sea) water and sand. First, we place the sand and salted water in the beaker. We separate the sand by filtering the mixture using the filter paper and funnel. Then we weigh the wet sand and we spread it on a tray until the water has evaporated. We weigh the dry sand.
Now we turn to the flask with the salted water. We weigh the flask with the salted water and place it in a warm place until the water has evaporated. We weigh the flask with the crystallised salt. Finally, we calculate the amount of salt and water in our sample.
| Flask + salted water + sand | Flask + salted water | Flask + sand | Wet sand | Dry sand | Amount of sand | Amount of water | |
| Weight [g] |
Part 3: Martian water
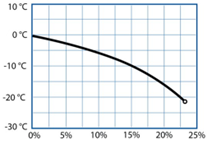
In 2018, the ESA Mars Express mission revealed some images that pointed to the existence of liquid water under the surface of Mars. The radar showed evidence of an area that could be a 20 km wide lake located 1.5 km under the surface of the Martian south pole.
Since the temperatures in this part of the planet can fall as low as ‑143 °C, scientists believe that the “water” (if present) must have high concentrations of salt in order to remain liquid.
In this part of the project, we will explore how the temperature at which water becomes ice, can be altered when mixed with other compounds. Alternatively, we can measure the time that different water and salt solutions take to freeze.
Materials
Beakers
Salt
Water
Freezer
Method
We make 6 solutions of water with different concentrations of salt going from pure water to 25% of salt in weight. We place the beakers in the freezer.
Students will observe that the presence of high amounts of salt in water prevents the water from freezing.





Part 4: The importance of water in astrobiology
Life on our planet evolved in primitive oceans; therefore, all living forms depend on water to survive. The water molecule is very versatile. It has such interesting properties that it is difficult to think of another substance in which life could have emerged.
The following experiments are designed to explore the properties of water.
Materials
Water
Plastic bottles
Freezer
Beaker
Method
We fill two bottles with the same amount of water, and we place one of them in the freezer. The following day, students can observe that the volume increases when water solidifies. If we fill a beaker with water and add a piece of ice, they also can observe that the level of water rises.
This is because water molecules separate from one another to acquire a certain pattern with fixed angles when water becomes ice.
In nature, this property is very important for aquatic organisms. During the winter months, the top layers of lakes and oceans freeze and because ice is less dense that liquid water, it floats, allowing life to continue under it.
This activity is done at home with the help of friends or family, followed by a discussion of the findings in class.
Method
At home, students measure the length of their hair or their nails before and after washing them.

Hydrogen bonds
The water molecule is made up of one oxygen atom and two hydrogen atoms. This molecule is polar, i. e. with partial positive electrical charges on the hydrogen atoms and a partial negative electrical charge on the oxygen atom. This causes the hydrogen atoms of some molecules to be attracted to the oxygen atoms of others, forming so-called hydrogen bonds. Hydrogen bonds are responsible for water having a high melting and boiling point.
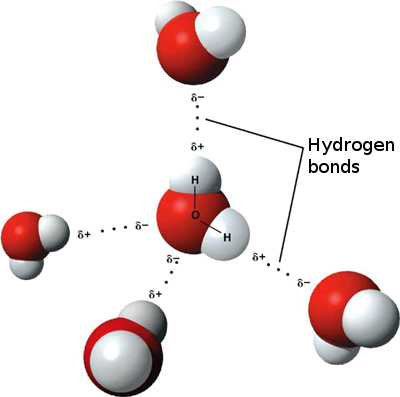
There are some proteins that form hydrogen bonds in their structure which make them more compact, such as the protein in hair and nails. These bonds can break easily with water. That is why our hair and nails are longer when they are wet.
Materials
Laboratory scale
Oven
Fruit and vegetables
Method
We weigh some fruit before and after placing them in the oven at 50 °C for 24 hours. We record the weight in a chart.
| Weight before [g] | Weight after [g] | % of water | |
| Tomato | |||
| Potato | |||
| Lettuce | |||
| Watermelon | |||
| Orange |
All living beings require water to fulfil their basic vital functions:
- Structural function
- Thermoregulatory function
- Solvent function
- Biochemical function
- Transport function
- Cushioning and lubricating function
We can calculate the percentage of water in different organisms by dehydrating them.
Materials
Different Coloured Paper
Mobile phone
Application to measure the intensity of light
Method
We place different coloured paper on a flat surface. We turn on the application and measure the amount of light reflected by the different colours with the mobile phone. The data is written in a chart.
| White | Yellow | Blue | Green | Red | Black | |
| Intensity |
The albedo effect
One of the most important effects for the planet is the albedo effect. Albedo is part of the Earth’s energy exchange and influences the Earth’s temperature but also climate change.
Albedo is a measure for the amount of energy reflected by a surface. Dark-coloured surfaces absorb most energy and reflect very little, so their albedo is close to zero. Pale-coloured surfaces, on the contrary, have high albedo as they reflect almost all of the energy. The energy that comes from the Sun is reflected by the atmosphere, clouds, and the Earth’s different surfaces (land, water, and ice). The amount of radiation reflected back from the Earth is called the planetary albedo.
There is a balance between the heat that is absorbed and the one returned to space, but this proportion can be altered by natural causes such as fires, hurricanes, volcanic eruptions and even plagues of insects that destroy vegetation. That is why the albedo effect plays a role in global warming. In areas of perpetual snow or ice, as in the poles, the albedo is very high because the snow and ice reflect practically all solar radiation, which contributes to decreasing the global temperature. Forests, on the other hand, have low albedo because the colour green absorbs a lot of radiation and therefore does not reflect almost any sunlight, thus contributing to increased warming in a certain area.
The albedo effect is part of the Earth’s energy balance, and both water and living beings are part of this unstable balance.
Material
Yellow, blue and red dye
3 beakers
Water
Two pieces of white cloth
Method
We dilute the dyes in water and place each colour in a beaker. We connect the beakers with the white cloths. The water will ascend through the cloth, and the colours will mix. This is due to capillarity.
Capillarity is a physical phenomenon that allows a liquid to rise to a certain height through very fine vessels. When a glass capillary tube is placed in a container of water, the water level will rise inside the tube. Now, if another tube with a larger diameter is introduced, the water that will enter it will show a lower level with respect to the narrower tube.
Plants absorb water and other substances from the soil through their roots. These substances are transported to the leaves by conductive vessels thanks to capillarity.





Conclusion and outlook
Astrobiology is a multidisciplinary science which covers various areas of traditional sciences. According to the NASA Astrobiology Institute, it focuses on three fundamental questions:
- How did life emerge and evolve?
- Does life exist in other parts of the universe?
- What is the future of life on Earth and beyond?
By using project-based learning, we complement the different topics contained in the curriculum with practical experiments.
We have found that this methodology
- is highly motivating,
- promotes skills development,
- allows students to design tasks based on the resolution of challenges,
- encourages socialization by exchanging knowledge in the classroom.
Astrobiology and science exploration are topics that permit the study of different areas of sciences cooperatively.
Books
Amigó, J. M., Ochando, L. E., Geologia i química del cosmos i de la Terra. Educació, Materials 51, Universitat de València, 2001, p. 568.
Fuente, A., El agua en el Universo. Observatorio Astronómico Nacional Instituto Geográfico Nacional. Ministerio de Fomento, 2012 (http://astronomia.ign.es/rknowsys-theme/images/webAstro/paginas/documentos/Anuario/elaguaeneluniverso.pdf).
Holland, H. D., The Chemical Evolution of the Atmosphere and Oceans. Princeton Series in Geochemistry, Princeton University Press, 1984, p. 582.
Hutchinson, S., Hawkins, L. E., Océanos. Bilbioteca Visual, Libros Cúpula, 2005, p. 305.
McMillan, B., Musick, J. A., Océanos. Larousse Editorial, 2007, p. 64.
Mottl, M., Glazer, B., Kaiser, R., Meech, K., Water and astrobiology. Chemie der Erde – Geochemistry, vol. 67, issue 4, 2007, pp. 253–282.
Rouchy, J.-M., Blanc-Valleron, M.-M., Les évaporites: matériaux singuliers, milieux extrêmes. Société Géologique de France et Vuibert, Collection “Interactions”, 2006, p. 190.
Online sources
https://elpais.com/ciencia/2020-08-27/el-misterio-del-origen-de-los-oceanos-terrestres.html
https://www.science.org/doi/full/10.1126/science.aba1948
https://weather.com/es-ES/espana/tiempo/news/2017-12-23-albedo-calentamiento-global-sol
La NASA confirma que en la Luna hay más agua de la que se creía (nationalgeographic.com.es)
Una sonda china mide 120 gramos de agua por tonelada de suelo lunar (europapress.es)
https://www.fundacionaquae.org/agua-hasta-en-el-cosmos/
https://www.elpuntocritico.com/vida-y-estilo/8-cultura/64130-agua-intergal%C3%A1ctica
https://www.ecologiaverde.com/funciones-del-agua-en-los-seres-vivos-3562.html
www.ecured.cu/Sustancias_moleculares
https://www.kyreo.es/la-mejor-agua-podemos-beber-la-contienen-frutas-verduras/
Templates for the tables used in activities 4, 5, 6, 10 and 11 are available in this Word document (download starts automatically).
Share this page